In this lab, you will determine the density of two different objects. Your data and results will be reported as two separate problems. You can write on the PDF file provided to you as a template, or you can write on your own sheets of paper.
Introduction and Theory
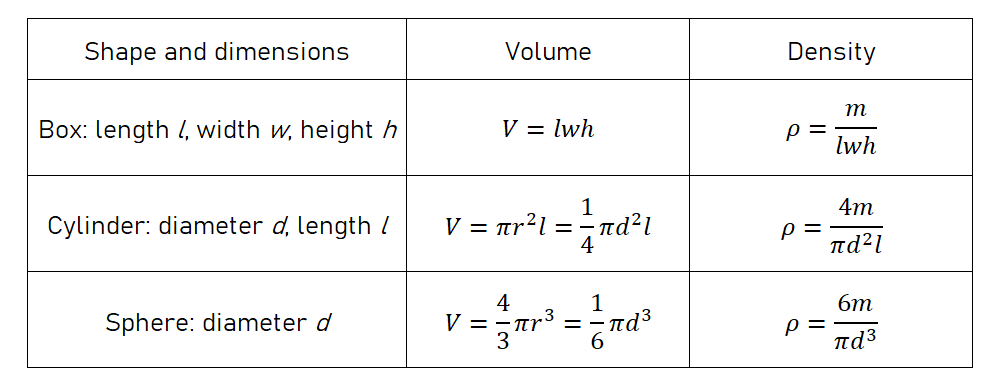
The density ρ of a solid object is defined as:
![]()
The standard unit of the density is g/cm3 or kg/m3. For example, the density of water is 1.00 g/cm3 or 1.00×103 kg/m3.
In this lab, we will find the density of two objects with regular shapes. For these objects, we can calculate the volume from the dimensions, and then calculate the density using mass over volume:
We will report the experiment in a student lab report and you must pay attention to the format. Write down your name, Langara student id, your 1114 section number, and the date you write the report at the top-left corner of the first page, then write the title of the report in the centre. The report must contain 5 sections for each problem: Purpose, Apparatus, Data, Calculations and Conclusion. It is recommended to have a Discussion section at the end of the entire report. Because this is your first real lab report, we will spell out some details and give examples to help you. They will not be reiterated, so you may want to revisit this page for later labs.
You must write in non-erasable ink but you can draw pictures or graphs in pencil. If you want to type out the entire report, you must attach your raw data, in ink, at the end of the report. It will not be part of the report, but to show that your data are genuine.
Problem 1
First, give a title to Problem 1. For example, you can write: “Problem 1, Density of butter".
Purpose
State the purpose or the goal of this experiment. For example, you can write: “Purpose: Determine the density of butter using mass over volume ".
Apparatus
List and/or provide photos of all the things you use in the experiment. You should provide enough information so that other people can repeat your experiment to check your result if needed. For example:

Data
Measure and record the mass and the dimensions of the object and record them in non-erasable ink. Make sure all data have: 1. descriptions; 2. units (use the units of the measuring tools); 3. proper sig figs and uncertainty. For example, you can record "The mass of the block of butter is (455 ± 5) g".
You may take only one reading for each quantity. For my example, because the shape of the butter block is not very regular, I measured each length three times at different places and then took the average. I organized these measurements in a data table:
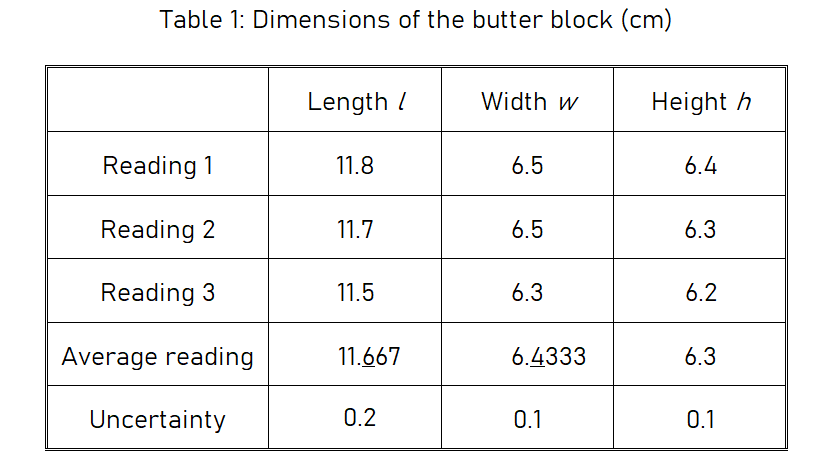
Calculations
At the beginning of the calculations, you should convert the units of the raw data to standard units. For this particular experiment, however, you may be able to skip some unit conversions if your data are in grams and centimeters, because g/cm3 is a common unit of the density. In fact, later we will see that it is the unit of our reference value, so using g/cm3 is far better.
Your calculations should have 3 steps: (1) Symbolic equation given by the theory; (2) Substituting the numbers with units; (3) The numerical result with units. Keep 5 non-zero digits for the results then round it to sig figs. For example:
![]()
Next you should compare your result to a commonly accepted value called the reference value, by calculating their percentage discrepancy, defined as:
![]()
For example: The reference density of butter, according to "Wikipedia: Butter", is 0.911 g/cm3. Therefore:
![]()
The digits in the percentage discrepancy are not related to the uncertainty, so they are not significant figures. One or two non-zero digits are enough to show the difference between the two values.
Conclusion
State your result with only the sig. figs. like below:
“The density of butter is found to be 0.95 g/cm3 which is about 4% higher than the reference density.”
Your conclusion in this and future experiments must meet the following requirements:
Problem 2 is done similarly, to measure the density of a different material.
Discussions
You may skip this section for this first try of writing lab report, but I will give a sample discussion to our example: "My result agrees with the reference value, given that my data have relatively big uncertainty. I may be able to get a better result if I use a more regular shaped block of butter, and remove the wraps."